| 山口 照英 |
- Yunoki M, Urayama T, Aoyama S, Okaniwa N, Sakai K, Uchida E, Ikuta K, Yamaguchi T. 2021. Polyethyleneimine-modified resins effectively remove porcine circovirus and cellular prion protein. J Virol Methods. 2021 Aug;294:114181
- Kang HN, Thorpe R, Knezevic I, Casas Levano M, Chilufya MB, Chirachanakul P, Chua HM, Dalili D, Foo F, Gao K, Habahbeh S, Hamel H, Kim GH, Perez Rodriguez V, Putri DE, Rodgers J, Savkina M, Semeniuk O, Srivastava S, Tavares Neto J, Wadhwa M, Yamaguchi T. 2021. Regulatory challenges with biosimilars: an update from 20 countries. Ann N Y Acad Sci. 1491:42-59.
- Yamaguchi T, Uchida E, Okada T, Ozawa K, Onodera M, Kume A, Shimada T, Takahashi S, Tani K, Nasu Y, Mashimo T, Mizuguchi H, Mitani K, Maki K. 2020. Aspects of Gene Therapy Products Using Current Genome-Editing Technology in Japan. Hum Gene Ther. 31:1043-1053.
- Kang HN, Thorpe R, Knezevic I; Survey participants from 19 countries including Yamaguchi T. 2020. The regulatory landscape of biosimilars: WHO efforts and progress made from 2009 to 2019. Biologicals. 65:1-9.
- Wadhwa M, Kang HN, Jivapaisarnpong T; WHO implementation workshop on guidelines on procedures and data requirements for changes to approved biotherapeutic products, Andalucia LR, Blades CDRZ, Casas Levano M, Chang W, Chew JY, Chilufya MB, Chirachanakul P, Cho HG, Cho YO, Choi KM, Chong S, Chua HM, Farahani AV, Gencoglu M, Ghobrial MRW, Guha P, Gutierrez Lugo MT, Ha SB, Habahbeh S, Hamel H, Hong Y, Iarutkin A, Jang H, Jayachandran R, Jivapaisarnpong T, Kang HN, Kim DY, Kim GH, Kim Y, Kwon HS, Larsen J, Lee AH, Lee J, Medvedeva K, Munkombwe Z, Oh I, Park J, Park J, Putri DE, Rodgers J, Ryu S, Savkina M, Schreitmueller T, Semeniuk O, Seo M, Shin YI, Shin J, Srivastava S, Song H, Song S, Tavares Neto J, Wadhwa M, Yamaguchi T, Youn HD, Yun M. 2020. WHO implementation workshop on guidelines on procedures and data requirements for changes to approved biotherapeutic products, Seoul, Republic of Korea, 25-26 June 2019. Biologicals. 65:50-59.
- Sakurai A, Ogawa T, Matsumoto J, Kihira T, Fukushima S, Miyata I, Shimizu H, Itamura S, Ouchi K, Hamada A, Tani K, Okabe N, Yamaguchi, T. 2019. Regulatory aspects of quality and safety for live recombinant viral vaccines against infectious diseases in Japan. Vaccine. 37:6573-6579.
- Furuta-Hanawa B, Yamaguchi T, Uchida E. 2019. Two-Dimensional Droplet Digital PCR as a Tool for Titration and Integrity Evaluation of Recombinant Adeno-Associated Viral Vectors. Hum Gene Ther Methods. 30(4):127-136.
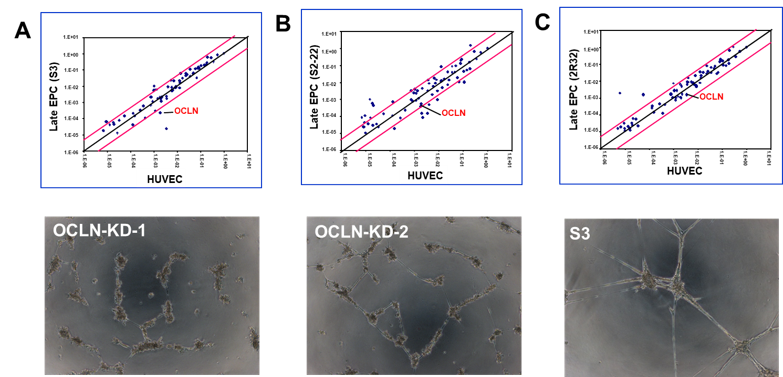
- Kanayasu-Toyoda,T., Ishii-Watabe,A., Kikuchi,Y. Kitagawa,H. Suzuk,H., Tamura,H., Tada,M., Suzuki,T., Mizuguchi,H, Yamaguchi,T:, Occludin as a functional marker of vascular endothelial cells on tube-forming activity. J Cell Physiol.233(2):1700-1711. (2018)
- Medina,R.J., Barber,C.L., Sabatier,L., Dignat-Grorge,F., Melero-Martin,J.M., Khosprotehrani,K., Phneda,O., RandiA.M., Chan,J.K.Y., Yamaguchi,T., Van Hinsberggh,V.W.M., Yoder,M.C., Stitt,A.W.; Endothelial Progenitors: A Consensus Statement on Nomenclature. Stem Cells Trans. Med. DOI: 10.1002/sctm.16-0360 (2017)
- Yamaguchi,T., Uchida,E.; Oncolytic Virus: Regulatory Aspects from Quality Control to Clinical Studies. Curr Cancer Drug Targets. doi: 10.2174/1568009617666170222142650. (2017)
|
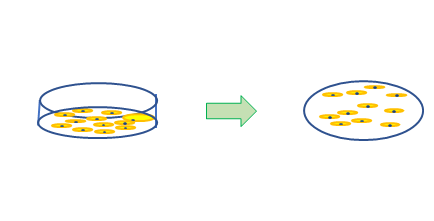 臍帯血から血管内皮前駆細胞を分離して
臍帯血から血管内皮前駆細胞を分離して 細胞を包埋してSCIDマウスに移植する
細胞を包埋してSCIDマウスに移植する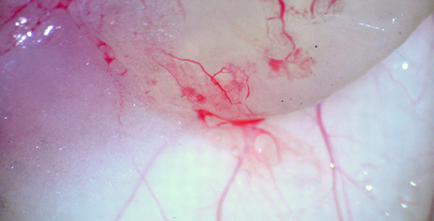 2, 3週間後に血管新生が起きる
2, 3週間後に血管新生が起きる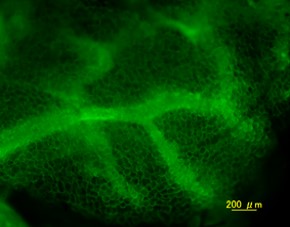 Tomato-Lectin-FITC
Tomato-Lectin-FITC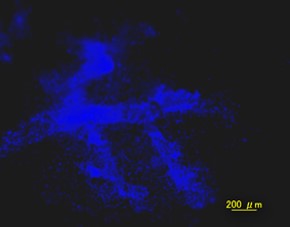 DAPI
DAPI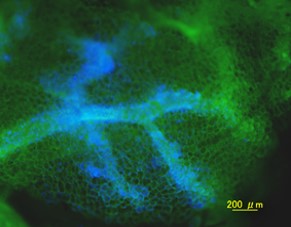 Merge
Merge